Consumables
Endoscope Decontamination
Chemistries and Consumables
Decontamination
Chemistries and Consumables
PRIORITISING PATIENT SAFETY
-
Endoscopy Decontamination Chemistries
FIND OUT MORERange of compliant, UK manufactured chemistries enabling effective endoscope decontamination.
-
Surgical Instrument Decontamination Chemistries
FIND OUT MOREUK manufactured chemistries to cover the effective decontamination of surgical instruments.
-
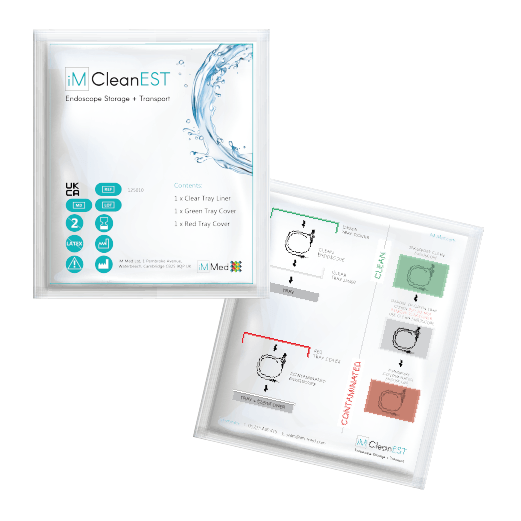
iM CleanEST: Endoscope Storage and Transportation
FIND OUT MOREClearly identify, store and transport decontaminated endoscopes and contaminated endoscopes and equipment with iM CleanEST.
-
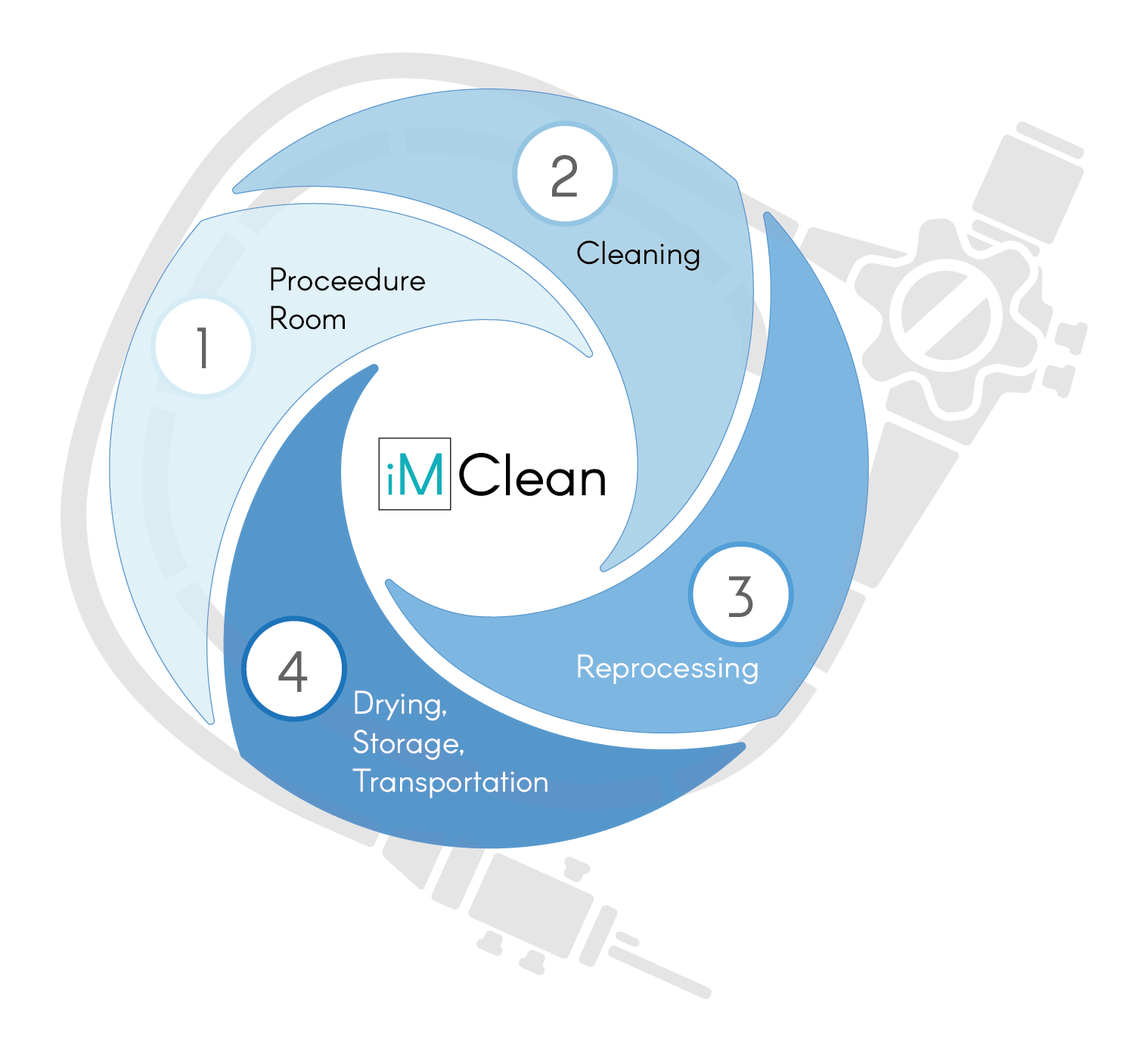
iM Clean Range
DISCOVER THE RANGECovering the decontamination journey of the endoscope, from use, through the decontamination stages, right back to the procedure room,
-
Endoscope Connectors and Accessories
FIND OUT MORECovering the vast majority of the scopes used in the UK.
-
Decontamination Consumables Catalogue
VIEW HERERange of consumables, connectors and decontamination equipment and services
-

“It’s a brilliant machine. Quick, easy and it extends the life of the scope”
Read the Gloucestershire Royal Hospital Case Study > -

“It’s a very simple process and easy to remember if you’ve not used the machine for some time. It’s very easy to train new staff. Everyone likes it.”
Read the Gloucestershire Royal Hospital Case Study >
THE DECONTAMINATION SPECIALISTS
Get the latest decontamination news and updates
Contact Us
Thank you for contacting us.
We will get back to you as soon as possible
We will get back to you as soon as possible
Oops, there was an error sending your message.
Please try again later
Please try again later